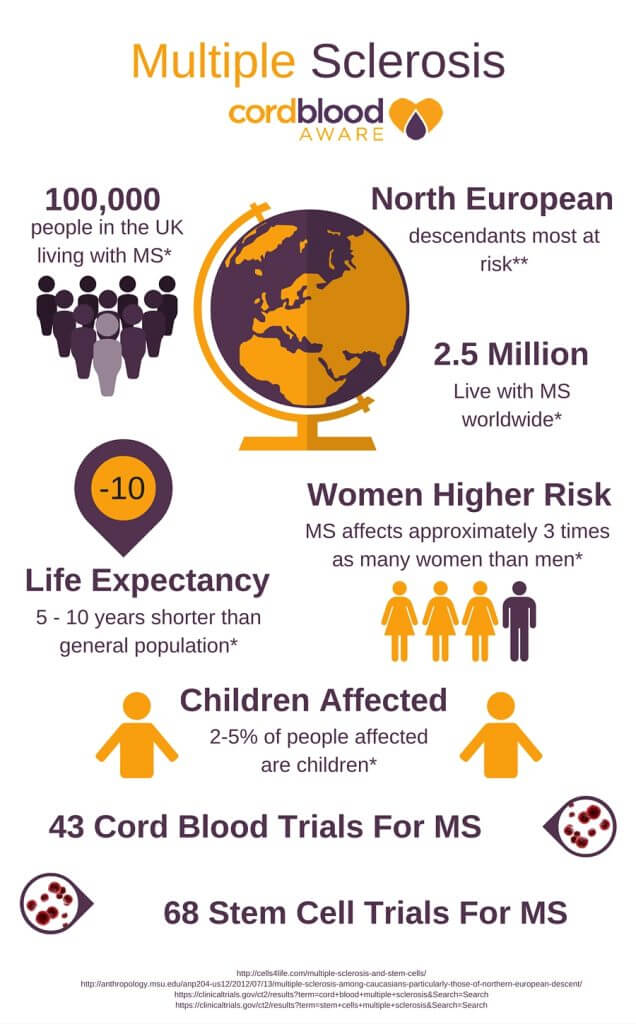
MS Stem Cell Trial in Transplant Breakthrough
MS Stem Cell Trial: Complete Remission After Transplant
A recent ms stem cell trial has been the first in the world to show complete long-term remission of ms.
However, the results of a recent clinical trial have given hope that a cure could soon be found. The trial, in Ottowa, used autologous haematopoietic stem cell transplants to reset the patient’s immune system.
Autologous stem cells come from the patient rather than a donor. Haematopoietic stem cells are found in the bone marrow, peripheral blood, and cord blood.
MS Stem Cell Transplant Research
Stem cell transplants have been used to treat cancers for years. However, the treatment is relatively new in the treatment of autoimmune conditions and is still under research. The long term efficacy of stem cell transplants in the treatment of multiple sclerosis is one of the reasons the treatment has not yet been made available.
MS Stem Cell Trial Shows Promising Results
The study in Canada followed 24 patients. Results showed 70% of patients who received stem cell therapy at least seven years ago saw the disease progression cease. Additionally, 40% of patients saw some symptoms, such as balance, vision, and muscle weakness, reverse [1].
Speaking about the ms stem cell trial, researcher Dr Harold Atkins said “our trial is the first to show the complete, long-term suppression of all inflammatory activity in people with MS. This is very exciting” [2].
The study results were met with praise and optimism with Dr Stephen Minger, stem cell biologist describing the trial results as “truly impressive” and “close to being curative” [3]. Professor John Snowden of Sheffield Teaching Hospitals took a more cautious approach and said “more clinical trials are needed to determine the ‘sweet spot’ – where the AHSCT technique is able to achieve a long term response in the majority of patients with an acceptable safety profile” [4].
The trial used peripheral blood as a stem cell source. Future stem cell research could also use umbilical cord blood and find another therapeutic use for this valuable resource.
You can read more about the trail in The Lancet.
[1] http://www.telegraph.co.uk/science/2016/06/09/multiple-sclerosis-patients-walking-working-and-skiing-after-gro/
[2] http://www.independent.co.uk/life-style/health-and-families/health-news/multiple-sclerosis-breakthrough-treatment-found-to-reverse-symptoms-a7073706.html
[3] http://multiplesclerosisnewstoday.com/blog/2016/06/13/stem-cell-ms-patients-lead-normal-life/
[4] https://www.generalandmedical.com/cms/news-media/posts/2016/june/new-treatment-may-stop-multiple-sclerosis-in-its-tracks/





